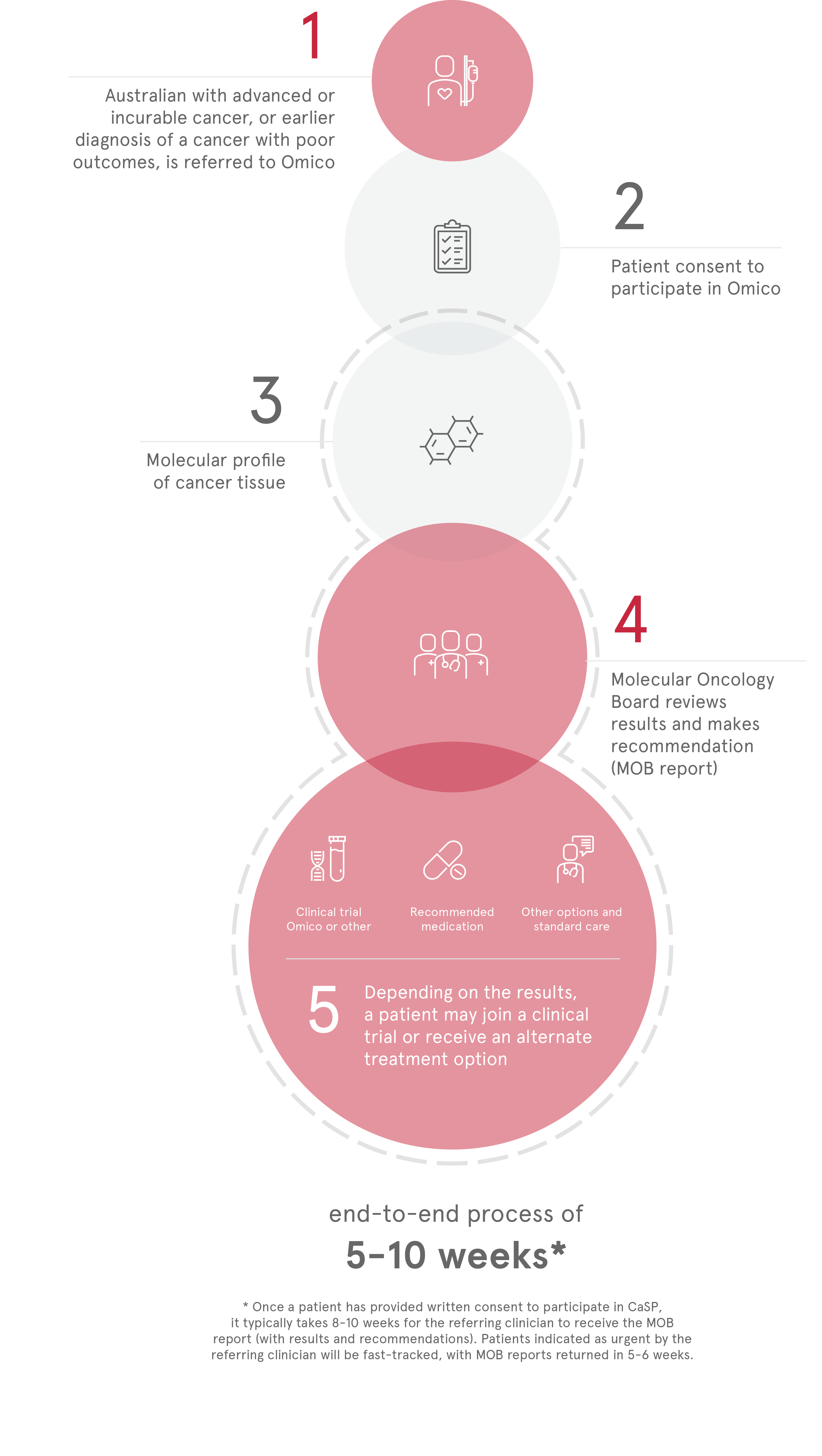
Standard biomarker screening
Traditionally, biomarker screening is performed in each individual clinical trial. This inefficient method contributes significant costs to running a clinical trial, costing in the region of AUD $10M to identify 200 trial participants.